UNCOVERING YTHDF2: A PROTEIN HELPING CANCER CELLS EVADE IMMUNOTHERAPY
By Pragathi and Ruchita
Introduction:
Immunotherapy is one of the popular methods for treating cancers, where the body’s immune system is triggered to fight off cancer cells. However, cancer cells are intelligent and can evolve mechanisms that can make them resistant to immunotherapy, thereby causing them to proliferate further and spread to different parts of the body, resulting in potential metastasis. Cancer cells can develop methods to hide from the immune system, like certain dormant HIV particles. One such biomarker, implicated in helping cancer cells conceal themselves, that has been identified by researchers is a protein called YTHDF2
Image: Crystal structure of N6-methyladenosine RNA reader YTHDF2
Source: YTHDF2 promotes ATP synthesis and immune evasion in B cell malignancies: Cell
Part 1:
The search for a cure to cancer is something that has persisted for centuries, and we still do not have a cure that can eradicate all types of cancers. Different therapies have been proposed as a part of the treatment process, such as radiotherapy, chemotherapy, viral therapy, immunotherapy, etc. Of these, immunotherapy has been shown to be a great alternative to conventional therapies as it harnesses the body’s natural immune system to target cancer cells. The immune system naturally detects and destroys oncogenic cells in healthy body conditions, but if the cancer progresses beyond a level where it starts becoming a burden to the body, the regular response produced by the immune system is not enough, requiring a more exaggerated response to target the infiltrating cancer cells. This approach has been greatly taken advantage of in many cases, and has drastically improved recovery and remission rates. Disease-free survival rates have jumped to 97.5% in the case of childhood and adult B-cell ALL. However, cancer cells are constantly evolving and developing a range of mechanisms to continue surviving and proliferating in the body. Cancer cells are successfully able to avoid immunological actions upon them by developing angiogenic mechanisms, mimicking healthy cells, producing immunosuppressive molecules and losing their ability to present tumor-specific antigens, which are required by the immune system to naturally recognise in order to initiate an immunological response. Therefore, there is a great need to discover cancer-specific biomarkers before cancer cells become resistant, which is one of the main aims of precision and personalised medicine techniques.
The central dogma of biology is pretty simple. Genes are transcribed to mRNA, a process known as gene expression or transcription, followed by the translation of the mRNA with the help of other RNA molecules like tRNAs and rRNAs into primary amino acid sequences, which fold into secondary, followed by tertiary and quaternary structures to give us proteins. Modifications occur at both a transcriptional and translational level, which can greatly impact the function of the protein molecules produced at the end, which are the fundamental building blocks of our body. These proteins are the units that again go on to regulate processes, such as transcription and translation.
The central dogma of biology is something that is essential to life itself, but we can exploit this very fact to innovate cancer therapies— mainly via targeting genes and manipulating their expression levels. Biotechnological breakthroughs in recent years have led to the identification of complex and unique biologic features associated with carcinogenesis, including genes and genetic markers. The treatment paradigm of cancer is slowly starting the shift from tumour-centered to more specific and personalised gene-directed, histology-agnostic treatments of precision medicine; next generation sequencing of advanced cancers has shown metastatic tumours harbour tremendously complex and unique genomic and immune landscapes, further highlighting the importance of precision medicine in the treatment of cancer.
Part 2:
YTHDF2 is one such gene that encodes a protein called YTH N6-methyladenosine RNA binding protein 2, which is a member of the YTH (YT521-B homology) superfamily containing the YTH domain. The YTH domain is usually located in the middle of the protein sequence and may function in binding to RNA. It is typical for eukaryotes and is particularly abundant in plants. It switches on genes that help cancer cells produce a stable energy source to fuel the cells' ability to grow and spread. Previously, its expression pattern has been confirmed in numerous previous studies, and its expression levels have been found to vary in different types of cancers, such as lymphomas, leukaemias, gastric, colon and pancreatic cancers, with a pattern of upregulation mostly seen. This protein helps cancer cells conceal themselves by reducing the presence of antigen biomarkers that normally trigger the immune system to detect and attack cancer.
YTHDF2, a highly effective m6A reader, specifically recognizes and degrades m6A-containing mRNAs in the cytoplasm, where it primarily resides. m6A readers are proteins that recognize and bind to m6A-modified RNA to regulate its fate. N6-methyladenosine (m6A) modification is one of the most common RNA modifications that involves three types of proteins, namely Writers (methyltransferases), which add the m6A modification to RNA, Erasers (demethylases), which remove the m6A modification and Readers, which recognize and interpret m6A-modified RNA. Tumor cells are able to manipulate m6A modifications to weaken the immune response, allowing them to grow undetected. Cancer cells use YTHDF2 to degrade immune-activating RNA that would normally help immune cells recognize and attack tumors. Some common modifications that can be induced are using writer proteins like METTL14 that interfere with immune signalling and eraser proteins such as FTO that help cancer cells to remove m6A markers, which support the expression of immune checkpoint genes that prevent immune cells from attacking, thereby helping cancer cells hide from the immune system. Such modifications mean fewer antigen-presenting molecules, so immune cells don’t get the "danger signal" they need to fight cancer. The upregulation of YTHDF2 is usually seen in the early CD8+ cytotoxic T cell activation phase, but if manipulated incorrectly due to modifications, it can reduce T cell effectiveness by altering their RNA metabolism. This leads to T cell exhaustion, meaning they lose their ability to kill tumors effectively.
Image: Biological pathway illustrating the mechanism of action of YTHDF2 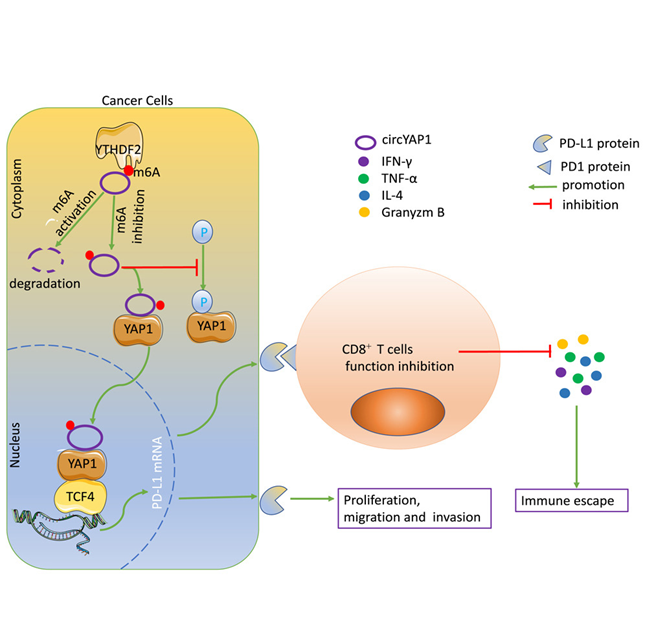
Source: https://www.cell.com/iscience/fulltext/S2589-0042(23)02856-0
Since YTHDF2 is showing to be a pan-cancer biomarker, many recent studies have been directed towards targeting it to reduce cancer proliferation and immune evasion. Scientists at City of Hope research institute, one of the largest and most advanced cancer research and treatment organizations in the U.S, in collaboration with its National Medical Center have created a new medicinal compound called CCI-38, which targets and suppresses YTHDF2, particularly in the case of leukemia and lymphoma patients receiving CAR T cell therapy, in cases where the cancer was becoming resistant to the therapy due to these modifications. CCI-38 is a small-molecule inhibitor. It selectively targets YTHDF2, enhancing CD19 expression, which is a protein that is expressed on B-lymphocytes and follicular dendritic cells, an indicator of B-cell malignancy and reduces overall tumor burden. By inhibiting YTHDF2, CCI-38 not only restores antigen expression but also reduces the energy supply to malignant cells, thereby enhancing the efficacy of CAR-T cell therapy. Another small molecule, DC-Y13-27, discovered by the Shanghai Institute of Materia Medica (SIMM), Chinese Academy of Sciences, has been shown to amplify the effects of radiotherapy and radio-immunotherapy, leading to better tumor control and reduced metastasis, in the case of solid tumors such as lung cancer, colorectal cancer
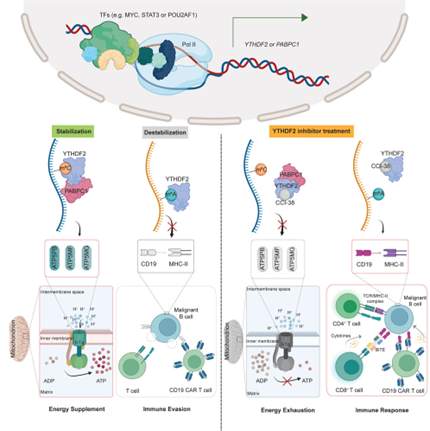 Image: Mechanism of action of CCI-38
Image: Mechanism of action of CCI-38
Source: YTHDF2 promotes ATP synthesis and immune evasion in B cell malignancies: Cell
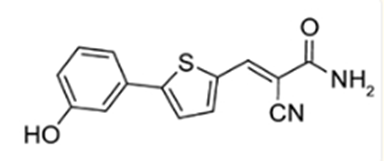
Image: Molecular structure of DC-Y13-27
Source: YTHDF2 inhibition potentiates radiotherapy anti-tumor efficacy - PMC
Part 3:
While YTHDF2 has shown to be a promising biomarker that can be targeted to reduce cases of immunotherapy resistance, there are still challenges to be addressed. YTHDF2 does not work all by itself, its effects depend on the interactions with other m6A “writers” (like METTL3, METTL14) and “erasers” (like FTO, ALKBH5), Therefore, the treatment must be designed exclusively looking at the specific reader, writer and eraser proteins being expressed. There exists a chance that targeting YTHDF2 may unintentionally affect other RNA-binding proteins, leading to unpredictable consequences in normal cells. Furthermore, the individual tumor microenvironments need to be taken into consideration. YTHDF2 can also be expressed in the case of healthy cells, and its inhibition might result in phenomena such as overexaggerated immune response or inflammatory systems, so it is important to take all these factors into consideration.
Conclusion:
In short, while YTHDF2 is undoubtedly a suitable biomarker that can be targeted for removing and preventing immune therapy resistance, further research needs to be done in order to develop a deeper understanding of its role in different cancers, identifying and creating more suitable selective inhibitors that can be used in patients with diverse writer and eraser protein combinations and improving translation from lab studies to clinical applications.
References:
- YTHDF2 inhibition potentiates radiotherapy anti-tumor efficacy - PMC
- Targeting YTHDF2 impacts the epitranscriptome and overcomes tumor therapy resistance: Trends in Cell Biology
- YTHDF2 promotes ATP synthesis and immune evasion in B cell malignancies: Cell
- YTHDF2 upregulation and subcellular localization dictate CD8 T cell polyfunctionality in anti-tumor immunity | Nature Communications
- Dynamic transcriptomic m6A decoration: writers, erasers, readers and functions in RNA metabolism | Cell Research
- YTHDF2-mediated circYAP1 drives immune escape and cancer progression through activating YAP1/TCF4-PD-L1 axis: iScience
- YTHDF2 in B Cell Malignancies: A New Pathway to Enhanced Immunotherapy | Inside Precision Medicine
- Scientists discover key protein that helps cancer cells evade CAR T cell therapy
- The tumor-intrinsic role of the m6A reader YTHDF2 in regulating immune evasion - PubMed
- YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8+ T cells | Nature Immunology
- The tumor-intrinsic role of the m6A reader YTHDF2 in regulating immune evasion | Science Immunology
- YTHDF2 Is a Therapeutic Target for HCC by Suppressing Immune Evasion and Angiogenesis Through ETV5/PD‐L1/VEGFA Axis - Wen - 2024 - Advanced Science - Wiley Online Library
- Review of precision cancer medicine: Evolution of the treatment paradigm